Movesense Medical’s MDR Registration Approaches – What Does It Mean for Movesense MD Customers?
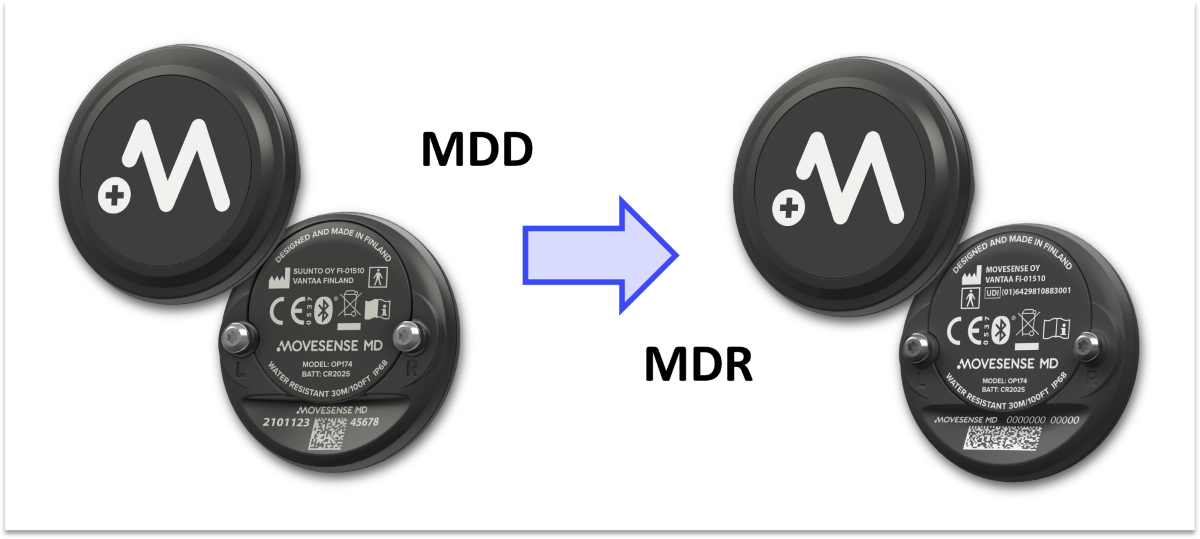
As communicated in our earlier news updates, we are going through the MDR conformity assessment of Movesense MD sensor. The new EU Medical Device Regulation 2017/745 (MDR) is replacing the Medical Device Directive MDD 93/42/EEC (MDD). Compliance with the MDR is mandatory for all new medical devices introduced to the EU market, and all existing devices must transition from the MDD to the MDR by May 2024.
The MDR registration process of Movesense MD is progressing well, and the transition is getting closer. This will also cause some changes to our customers. The exact transition date is still open but there are a few things that can be prepared already.
What changes?
The regulation: Movesense MD will conform to the MDR instead of the MDD.
Product status: According to the MDR, Movesense MD will be listed as medical device, not as a medical device accessory like currently under the MDD.
Under the MDR, a medical device accessory is always intended to be used with a specific, named host device. As a medical device, Movesense MD can be used with any other medical device for the intended use of ECG and movement measurement. In practice, however, the impact of this change to Movesense customers is minimal.
Manufacturer: Currently, the manufacturer of Movesense MD is Suunto, and Movesense Ltd acts as a distributor. This arrangement goes back to Movesense’s previous ownership under Suunto. Once the MDR registration exists, Movesense becomes the manufacturer of Movesense MD. As a result, also the product label of Movesense MD will list Movesense Ltd as the manufacturer of the product. This includes the laser marking on the back cover of the device, product packaging, instructions for use, and other items of the product label.
What doesn’t change?
The device: The actual sensor stays unchanged except for the laser printing on the back cover that will get the new manufacturer name and the Unique Device Identification number. There are no changes in the technical specifications of the sensor. Download Movesense MD datasheet.
Developing: Nothing related to the technical features of the sensor will change. Programming, development tools, firmware versions, and technical capabilities stay the same. The most recent Movesense Medical firmware version is 2.1.3. The next medical firmware version will become available independent of the regulation change. View developer resources.
Movesense relations: all contact persons, commercial conditions, and communication with the team will continue as before.
What does the change mean for Movesense customers?
1. Customers developing a new health solution using Movesense MD
If you are working on an application that processes Movesense MD sensor data to provide a clinical benefit, and plan to bring it to the market in EU, you will have to certify your solution as a medical device according to the MDR.
This includes verifying and validating that your device works together with Movesense MD, and listing Movesense MD as a compatible device. You can then certify the solution according to the MDR as a stand-alone or as a system/procedure pack, depending on how the system components are delivered to customers.
The upcoming certification will prove that Movesense MD and its default firmware comply with the MDR. If you are developing your own sensor firmware, it is not covered with the MDR registration of Movesense MD. You must validate the possible clinical benefit and certify the solution separately.
We warmly recommend finding a local external MDR expert to support you in the certification process. This is especially useful if you haven’t done such a registration before. Movesense will support you by providing the necessary documentation of the sensor.
If you are already using the MDD registered Movesense MD sensor for development or testing purposes, you can continue your development project normally. The device is technically identical to the upcoming Movesense MD (MDR). Just take the change into account in your possible documentation.
The MDR compliant Movesense Medical sensor will be available withing next few months. We will update the exact date soon. Please contact orders@movesense.com, if you want to place pre-orders.
2. Customers currently using Movesense MD, class II a medical device accessory (MDD) with a host device or an application
LAST TIME BUY APPLIES
The current MDD registration of Movesense MD will remain applicable during the regulative transition, and the products can be sold as part of your solution until the end of the MDD transition period. After this, you will have to start using the MDR registered sensor variant. Products sold to users can be used normally until the end of their lifetime.
However, we will ramp down the capability to provide products under the MDD registration after receiving the MDR certification. Therefore, the last time buy for the MDD registered sensors is February 28, 2023. This is the date when we need your binding orders. The MDD version is available while stock lasts.
A change of device manufacturer can be seen as a change of a critical supplier, which is regarded as a significant change. Please check how your own ISO 13485 quality management system defines your change control activities in such a situation, and plan and implement the change accordingly.
3. Customers planning to use out-of-the-box sensor data of Movesense MD (MDR)
If you are integrating sensor data provided by Movesense MD’s default firmware, such as ECG or heart rate, into an existing, CE marked digital health data system or software, you can utilize the data quickly.
In such cases, no additional MDR registration of the sensor and software combination is required, which speeds up the deployment considerably. It is sufficient that you verify that Movesense MD meets the requirements of your software, test that they work together as expected, and list Movesense MD as a compatible device with your software.
A new compatible device is usually considered a significant change and you must inform your notified body about it. Such a change is usually very software-specific, so the software manufacturer is the best party to assess the actual impact.
Another acceleration lane to the market without a new MDR registration is bundling Movesense MD with another CE marked medical device as described in the MDR article 22, Systems and procedure packs.
To create a system of Movesense MD and another device bearing the CE marking, you will have to verify the compatibility of the devices and make a statement to declare the compatibility. In addition, you will need to provide instructions for use specific to the combined use of the devices, register the system in EUDAMED database, and get a new Unique Device Identification for the system.
However, we recommend always confirming the situation with your specific solution with your notified body before going to market.
4. Non-EU customers
The MDR registration covers the EU countries and is accepted by some other countries. However, many countries have their own regulation for medical device registration, such as the FDA regulation in the U.S. We are happy to support the local medical registration of your Movesense powered solution by providing you with all the necessary documentation related to the sensor.
Further information
Movesense team is happy to answer any questions related to the change and available for meetings if needed. You can always reach us via info@movesense.com and by getting in touch with your current contact person.
Views: 121
