Description
Movesense MD is a Bluetooth-equipped, Class IIa (MDR) wearable medical device, and it is intended for medical applications in healthcare and remote patient monitoring. This Movesense MD Developer kit is intended for getting started with the technical development with it.
The programmable sensor is designed for measuring ECG, heart rate, and movement. It can be custom branded. It runs on an open API, allowing the development of use-case-specific firmware for customers’ solutions.
Movesense Medical sensor is registered as a Class IIa medical device according to MDR 2017/745 and manufactured under ISO 13485 quality system.
The kit includes free access to Movesense developer tools, sample code, and documentation. Developed, designed and manufactured in Finland.
Kit Content
- Movesense Medical sensor
- Accessories:
- 1 pcs Heart Rate Monitor Belt with Movesense connector (black, size M)
- 1 kit of ECG Glue-on Patch Electrode (2 x ECG patch)
- 1 pcs Movesense Wrist Mount
- 1 pcs Movesense Clothing Clip
- 1 pcs Movesense Surface Mount
Please contact sales@movesense.com for business inquiries and clinical usage.
Please note that the Movesense MD development kit documentation is available in English language only.
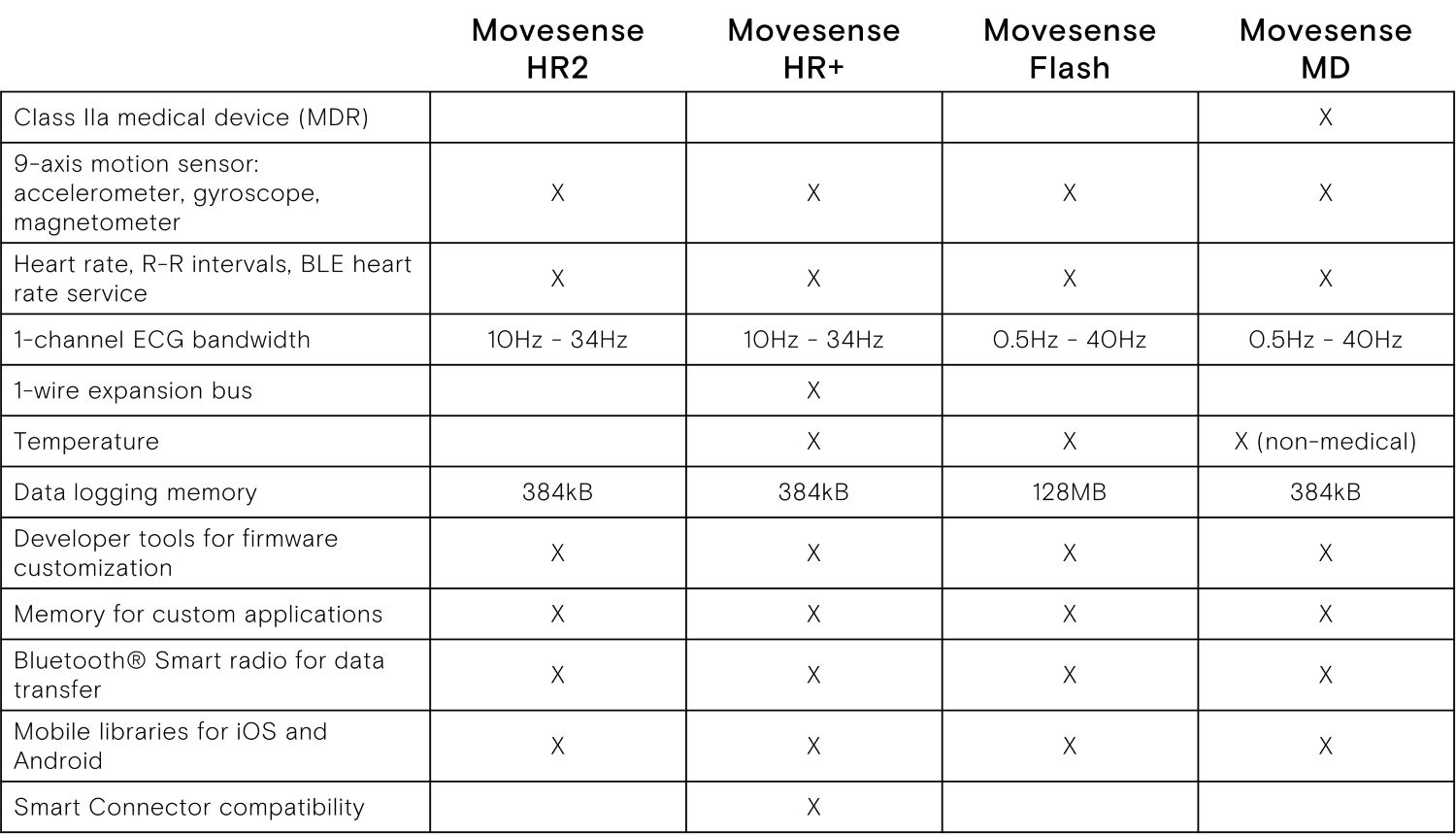
Technical highlights
- Single channel ECG, Heart rate, R-R intervals
- 9-axis movement sensor: 3 x accelerometer,
3 x gyroscope, 3 x magnetometer - Configurable sample rates
- In-built memory for logging data
- Bluetooth® Smart compatible
- Software tools for developing applications that run on the sensor
- Wireless firmware update with BLE
- Water (IP68) and shock proof construction, suitable for health and medical applications.
- Low profile snap connection for attaching the sensor to apparel and gear.
- User replaceable battery (CR2025 coin cell)
- Battery life optimization options through software.
- Weight 9.4 g / 0.33 oz. with battery, diameter 36.6 mm / 1.44 inches
Developing medical applications with Movesense MD
Movesense MD firmware is developed in accordance with
IEC 62304: 2006 standard.
Read more about using Movesense MD for healthcare and medtech needs.
For experimenting and quick trials, you can use the non-medical firmware that is fully compatible with the sensor.
When starting to develop a commercial medical firmware, please send an email to info@movesense.com to receive access to the medical firmware development repository.
For feedback on Movesense Medical sensor, please use email medical@movesense.com.
At this point the Movesense Medical ECG Developer Kit is available for business customers only.
Views: 852