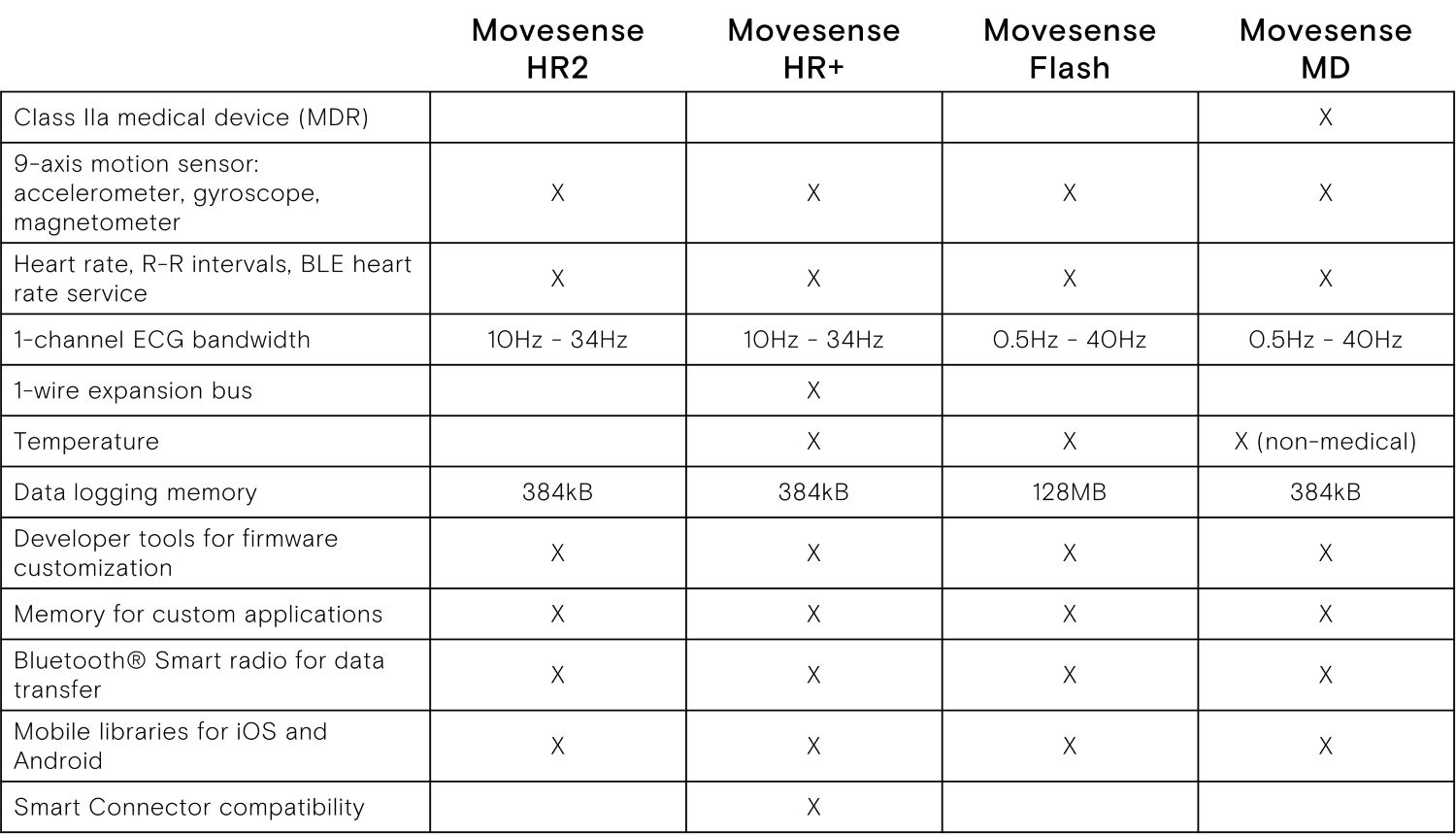
Description
- Designed for medical applications and remote patient monitoring
- Specialty: Class IIa medical device according to MDR 2017/745
- Manufactured under ISO 13485 quality management system
- Use as a medically certified heart rate monitor, via industry standard Bluetooth Heart Rate Service
- Differences from other Movesense sensors, please see the picture above
- The sensor is equipped with standard firmware that can be customized to meet your needs
- Free use of Movesense developer tools and sample code
- Full access to raw data
- Also available as a white-labeled OEM product
- Developed, designed and manufactured in Finland
- Movesense has a NATO NCAGE code for participation in respective tenders
Please contact sales@movesense.com for business and volume pricing inquiries and clinical usage.
Please note that currently the Movesense MD sensor documentation is available in English language only.
Technical highlights
- Single channel ECG, Heart rate, R-R intervals
- 9-axis movement sensor: 3 x accelerometer,
3 x gyroscope, 3 x magnetometer - Configurable sample rates
- In-built memory for logging data
- Bluetooth® Low Energy (BLE)
- Software tools for developing applications that run on the sensor
- Wireless firmware update with BLE
Download Movesense Medical data sheet and User Guide
- Water (IP68) and shock proof construction, suitable for medical, health and sports applications
- Developed, designed and manufactured in Finland
- Suitable for ECG and movement signal registration in oxygen rich environment, such as hyperbaric chambers
- User replaceable battery (CR2025 coin cell)
- Battery life optimization options through software.
- Weight 9.4 g/0.33 oz. with battery, diameter 36.6 mm/1.44 in, body thickness 8 mm/0.32 in
Developing medical WEARABLES AND HEALTH applications with Movesense Medical
Movesense MD firmware is developed in accordance with
IEC 62304: 2006.
Read more about using Movesense MD for healthcare and medtech needs.
For experimenting and quick trials, you can use the non-medical firmware that is fully compatible with the sensor.
When starting to develop a commercial medical firmware, please send an email to info@movesense.com to receive access to the medical firmware development repository.
For feedback on Movesense Medical sensor, please use email medical@movesense.com.
At this point the Movesense Medical is available for business customers only,