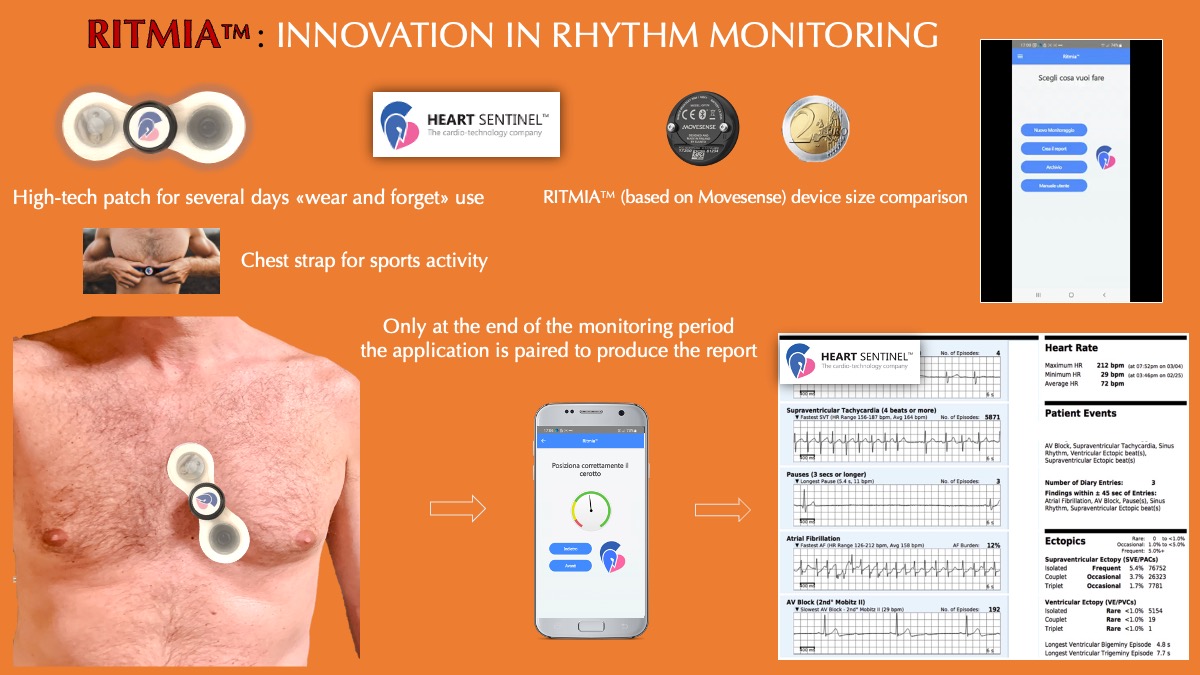
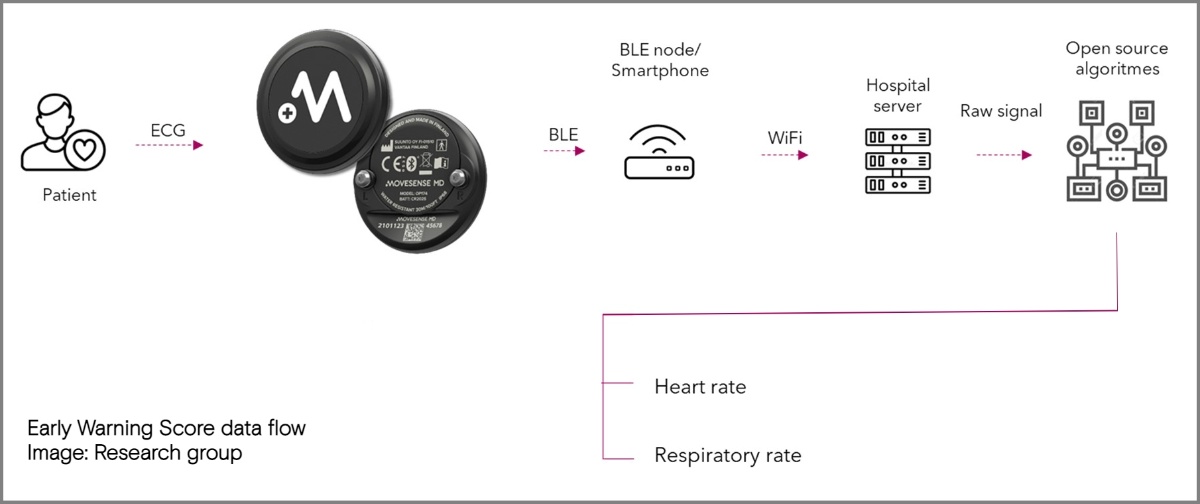
HeartSentinel Launches Ritmia, an Automatic Arrhythmia Detection Tool
Heart Sentinel srl is a biotech company founded in 2016 in Parma, Italy by Nicola Gaibazzi and Claudio Reverberi, recognized Italian cardiologists and authors of several key scientific publications related to cardiovascular diseases. The company now dedicates to develop and patent smart products in the field of cardiology, using Movesense devices. Their aim is to democratize long-term cardiac rhythm monitoring […]